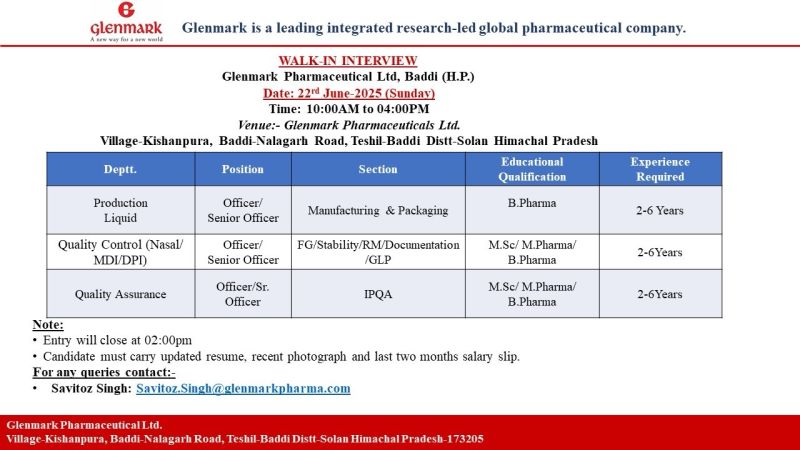
Glenmark- Walk-In Interview For QC/QA & Production on 22nd June 2025
Date: Sunday, 22nd June 2025
Time: 10:00 AM to 4:00 PM (Entry closes at 2:00 PM)
Venue: Glenmark Pharmaceuticals Ltd., Village-Kishanpura, Baddi-Nalagarh Road, Tehsil-Baddi, Distt-Solan, Himachal Pradesh – 173205
1. Production – Liquid
Position: Officer / Sr. Officer
Section: Manufacturing & Packaging
Qualification: B. Pharm
Experience: 2–6 Years
2. Quality Control
Position: Officer / Sr. Officer
Sections: FG, Stability, RM, Documentation, GLP
Qualification: M.Sc / M. Pharm / B. Pharm
Experience: 2–6 Years
3. Quality Assurance
Position: Officer / Sr. Officer
Section: IPQA
Qualification: M.Sc / M. Pharm / B. Pharm
Experience: 2–6 Years
Please Carry:
- Updated Resume
- Recent Passport-size Photograph
- Last 2 Months’ Salary Slips
Contact for Queries: [email protected]
Job Category: Pharma
Job Type: Full Time
Job Location: Baddi