EVALUATION ACTIVITIES – THE QUALITY SYSTEMS MODEL
The elements of a quality system correlate closely with the requirements in the CGMP regulations.
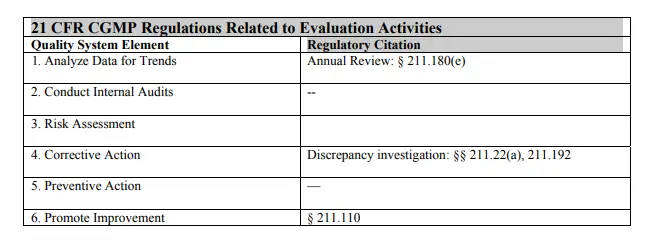
1. Analyze Data for Trends of QUALITY SYSTEMS MODEL
Quality systems call for continually monitoring trends and improving systems. This can be achieved by monitoring data and information, identifying and resolving problems, and anticipating and preventing problems.
Quality systems procedures involve collecting data from monitoring, measurement, complaint handling, or other activities, and tracking this data over time, as appropriate. Analysis of data can provide indications that controls are losing effectiveness. The information generated will be essential to achieving problem resolution or problem prevention (see IV.D.3.).
Although the CGMP regulations (§ 211.180(e)) require product review on at least an annual basis, quality systems approach calls for trending on a more frequent basis as determined by risk. Trending enables the detection of potential problems as early as possible to plan corrective and preventive actions. Another important concept of modern quality systems is the use of trending to examine processes as a whole; this is consistent with the annual review approach.
Trending analyses can help focus internal audits
2. Conduct Internal Audits of QUALITY SYSTEMS MODEL
A quality systems approach calls for audits to be conducted at planned intervals to evaluate the effective implementation and maintenance of the quality system and to determine if processes and products meet established parameters and specifications. As with other procedures, audit procedures should be developed and documented to ensure that the planned audit schedule takes into account the relative risks of the various quality system activities, the results of previous audits and corrective actions, and the need to audit the complete system. Procedures should
describe how auditors are trained in objective evidence gathering, their responsibilities, and auditing procedures. Procedures should also define auditing activities such as the scope and methodology of the audit, selection of auditors, and audit conduct (audit plans, opening meetings, interviews, closing meetings, and reports). It is critical to maintain records of audit findings and assign responsibility for the follow-up to prevent problems from recurring (see IV.D.3.).
The quality systems model calls for managers who are responsible for the areas audited to take
timely action to resolve audit findings and ensure that follow-up actions are completed, verified,
and recorded. (FDA’s policy is to refrain from both reviewing and copying reports or records
that result from internal audits per Compliance Policy Guide 130.300.19).
3. Quality Risk Management of QUALITY SYSTEMS MODEL
Effective decision-making in a quality systems environment is based on an informed understanding of quality issues. Elements of risk should be considered relative to the intended use of a product and in the case of pharmaceuticals, patient safety and ensuring the availability of medically necessary drug products. Management should assign priorities to activities or actions based on an assessment of the risk including both the probability of occurrence of harm and of the severity of that harm. It is important to engage appropriate parties in assessing the risk. Such
parties include customers, appropriate manufacturing personnel, and other stakeholders.
Implementation of quality risk management includes assessing the risks, selecting and implementing risk management controls commensurate with the level of risk, and evaluating the results of the risk management efforts. Since risk management is an iterative process, it should be repeated if new information is developed that changes the need for, or nature of, risk management.
In a manufacturing quality systems environment, risk management is used as a tool in the development of product specifications and critical process parameters. Used in conjunction with process understanding, quality risk management helps manage and control change.
4. Corrective Action of QUALITY SYSTEMS MODEL
Corrective action is a reactive tool for system improvement to ensure that significant problems do not recur. Both quality systems and the CGMP regulations emphasize corrective actions.
Quality systems approaches call for procedures to be developed and documented to ensure that the need for action is evaluated relevant to the possible consequences, the root cause of the problem is investigated, possible actions are determined, a selected action is taken within a defined timeframe, and the effectiveness of the action taken is evaluated. It is essential to document corrective actions taken (CGMP also requires this; see § 211.192).
It is essential to determine what actions will reduce the likelihood of a problem recurring.
Examples of sources that can be used to gather such information include the following:
- Nonconformance reports and rejections
- Returns
- Complaints
- Internal and external audits
- Data and risk assessment related to operations and quality system processes
- Management review decisions
5. Preventive Actions
Being proactive is an essential tool in quality systems management. Succession planning, training, capturing institutional knowledge, and planning for personnel, policy, and process changes are preventive actions that will help ensure that potential problems and root causes are identified, possible consequences assessed, and appropriate actions considered.
The selected preventive action should be evaluated and recorded, and the system should be monitored for the effectiveness of the action. Problems can be anticipated and their occurrence prevented by reviewing data and analyzing risks associated with operational and quality system processes, and by keeping abreast of changes in scientific developments and regulatory requirements.
6. Promote Improvement of QUALITY SYSTEMS MODEL
The effectiveness and efficiency of a quality system can be improved through the quality activities described in this guidance. Management may choose to use other improvement activities as appropriate. It is critical that senior management be involved in the evaluation of this improvement process (see IV.D.3.).
The following table shows how the CGMP regulations correlate to specific elements in the quality systems model for this section. Manufacturers should always refer to specific regulations to make sure they are complying with all regulations.