Disintegration Test in Pharma Industry
This test determines whether dosage forms such as tablets, capsules, boluses pessaries, and suppositories disintegrate
within a prescribed time when placed in a liquid medium under the prescribed experimental conditions.
For the purpose of this test, disintegration does not imply complete solution of the dosage unit or even of its active
constituent. Disintegration is defined as that state in which no residue of the unit under test remains on the screen of the apparatus or, if a residue remains, it consists of fragments of disintegrated parts of tablets component parts such as insoluble coating of the tablets or of capsule shells, or of any melted fatty substance from the pessary or suppository or is a soft mass with no palpable core. If discs have been used with capsules, any residue remaining on the lower surfaces of the discs consists only of fragments of shells.
For tablets and capsules in Pharma Industry
Apparatus
The apparatus consists of a basket-rack assembly, a 1-liter beaker, a thermostatic arrangement for heating the fluid and a mechanical device for raising and lowering the basket in the immersion fluid at a constant frequency rate.
Basket-rack assembly.
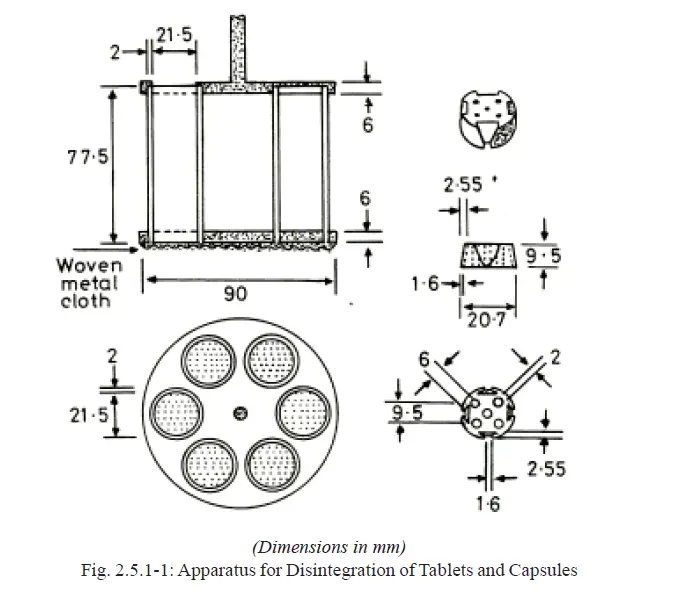
The basket-rack assembly is rigid and supports six cylindrical glass tubes, 77.5 ± 2.5 mm long, 21.5 mm in internal diameter and with a wall thickness of about 2 mm (Fig. 2.5.1-1).
The tubes are held vertically by two superimposed transparent plastic plates, 90 ± 2 mm in diameter and 6.75 ± 1.75 mm thick perforated by six holes having the same diameter as the tubes.
The holes are equidistant from the centre of the plate and are equally spaced from one another.
Attached to the underside of the lower plate is a woven stainless steel wire cloth with a plain square weave with 2.0 ± 0.2 mm mesh apertures and with a wire diameter of 0.615 ± 0.045 mm. The upper plate is covered with a stainless steel disc perforated by six holes, each about 24 ± 2 mm in diameter, which fits over the tubes and holds them between the plastic plates. The holes coincide with those of the upper plastic plate and the upper open ends of the glass tubes.
A suitable means is provided to suspend the basket-rack assembly from the raising and lowering device using a point on its axis.
The plates are held rigidly in position and 77.5 mm apart by vertical metal rods at the periphery and a metal rod is also fixed to the centre of the upper plate to enable the assembly to be attached to the device for raising and lowering it smoothly at a constant frequency of between 28 and 32 cycles per minute through a distance of 50 to 60 mm.
The time required for the upward stroke is equal to the time required for the downward stroke, and the change in stroke direction should be smooth and not abrupt.
There should be no appreciable horizontal motion or movement of the axis from the vertical.
The design of the basket-rack assembly may be somewhat different provided specifications for the glass tubes and the screen mesh size are unchanged.
Discs.
A cylindrical disc for each tube, each 20.7 ± 0.15 mm thick in diameter and 9.5 ± 0.15 mm thick, made of transparent plastic with a relative density of 1.18 to 1.20, and pierced with five holes, each 2 mm in diameter, one in the centre and the other four spaced equally on a circle of radius 6 mm from the centre of the disc. Four equally-spaced grooves are cut in the lateral surface of the disc in such a way that at the upper surface of the disc they are 9.5 mm wide and 2.55 mm deep and at the lower surface 1.6 mm square.
Medium.
The assembly is suspended in the liquid medium in a suitable vessel, preferably a 1-litre beaker.
The volume of liquid is such that the wire mesh at its highest point is at least 25 mm below the surface of the liquid, and at its lower point is at least 25 mm above the bottom of the beaker.
At no time should the top of the basket-rack assembly become submerged.
There is a thermostatic arrangement for heating the liquid and maintaining the temperature at 37º ± 2º.
Method.
Unless otherwise stated in the individual monograph, introduce one tablet or capsule into each tube and, if directed
in the appropriate general monograph, add a disc to each tube.
Suspend the assembly in the beaker containing the specified liquid and operate the apparatus for the specified
time. Remove the assembly from the liquid.
The tablets or capsules pass the test if all of them have disintegrated.
If 1 or 2 tablets or capsules fail to disintegrate, repeat the test on 12 additional tablets or capsules; not less than 16 of the total of 18 tablets or capsules tested disintegrate.
If the tablets or capsules adhere to the disc and the preparation under examination fails to comply, repeat the test omitting the disc.
The preparation complies with the test if all the tablets or capsules in the repeat test disintegrate.
For enteric-coated tablets in Pharma Industry
Apparatus.
Use the apparatus for tablets and capsules described above.
Method.
Put one tablet into each tube, suspend the assembly in the beaker containing 0.1M hydrochloric acid and operate
without the discs for 2 hours, unless otherwise stated in the individual monograph. Remove the assembly from the liquid.
No tablet shows signs of cracks that would allow the escape of the contents or disintegration, apart from fragments of
coating.
Replace the liquid in the beaker with mixed phosphate buffer pH 6.8, add a disc to each tube and operate the apparatus for a further 60 minutes.
Remove the assembly from the liquid. If the tablet fails to comply because of adherence to the disc, repeat the test on a further 6 tablets without the discs.
The tablets pass the test if all six have disintegrated.
For pessaries and suppositories in Pharma Industry
Apparatus
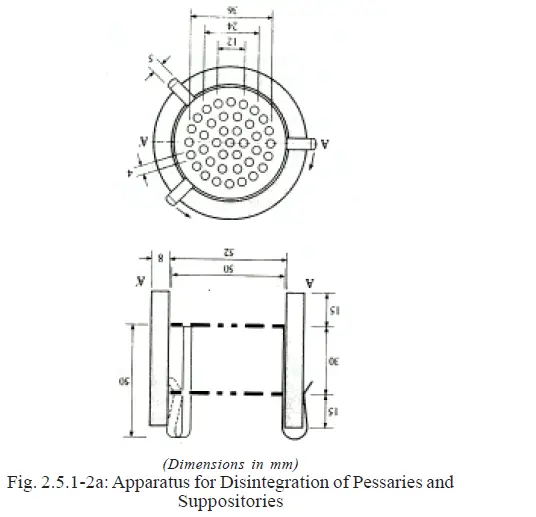
a) A transparent sleeve of glass or plastic, 60 mm high with an internal diameter of 52 mm and an appropriate wall
thickness (Fig.2.5.1-2a).
b) A metal device consisting of two stainless steel discs each of which contains 39 holes, each 4 mm in diameter,
being distributed as indicated in Fig 2.
The diameter of the disc is closely similar to the internal diameter of the sleeve. The discs are separated by a distance of about 30mm.
The metal device is attached to the outer sleeve by means of three equally spaced hooks.
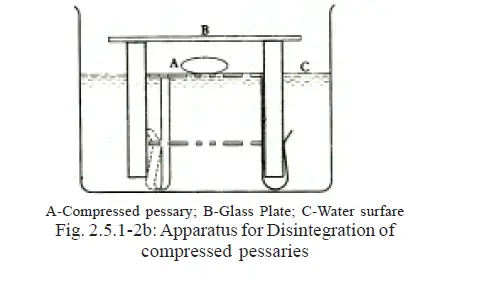
For Compressed Pessaries use with the hook-end downwards as in Fig. 2.5.1-2b.
For Moulded Pessaries, Moulded Suppositories, Shell Pessaries and Shell Suppositories
Place a pessary or suppository on the lower perforated disc of the metal device and then insert the device into the cylinder and attach this to the sleeves.
Repeat the operation with a further two pessaries or suppositories and metal devices and sleeves.
Unless otherwise specified, place each piece of apparatus in a vessel containing at least 4 litres of water at 36º
to 37º and fitted with a slow stirrer and a means of holding the top of the apparatus 90mm below the surface of the water.
A suitable thermostatic arrangement may be provided for maintaining the temperature of the bath. Alternatively, all three pieces of apparatus may be placed together in a vessel containing at least 12 litres of water.
After each 10 minutes invert each apparatus without removing it from the liquid.
Disintegration is considered to be complete when the moulded pessary or suppository
a) is completely dissolved or
b) has dispersed into its component parts, which may remain on the surface (in the case of melted fatty substances),
sink to the bottom (in case of insoluble powders) or dissolve (in case of soluble components) or maybe
distributed in one or more of these ways or
c) has become soft with appreciable change in shape, without necessarily separating into its components, and
the mass has no solid core which cannot be pressed with a glass rod.
For Compressed Pessaries in Pharma Industry
Place the apparatus in a vessel of suitable diameter containing water at 36º to 37º C.
Adjust the level of the liquid by the gradual addition of water at 36º to 37º until the perforations in the metal disc are just covered by a uniform layer of water.
Place one compressed pessary on the upper perforated disc and cover the apparatus with a glass plate to ensure a humid atmosphere.
Repeat the operation with a further two compressed pessaries.
Disintegration is considered to be complete when
a) there is no residue on the perforated plate or
b) if a residue remains, it consists only of a soft mass having no solid core which cannot be pressed with a glass rod.
Effectiveness of Antimicrobial Preservatives in Pharma industry