Enhancing Efficiency with Automatic Rotary Type Measuring Cup Placement In the fast-paced world of manufacturing, efficiency and accuracy are crucial factors for success. Automatic rotary type measuring cup placement is a cutting-edge technology that revolutionizes the process of measuring and placing cups onto containers or packaging in various industries. This …
Read More »Revolutionizing Product Packaging with Bottle Sticker Labelling Machines
Revolutionizing Product Packaging with Bottle Sticker Labelling Machines In today’s competitive market, effective product packaging plays a crucial role in capturing consumer attention and promoting brand recognition. The bottle sticker labelling machine is a cutting-edge technology that streamlines the labelling process for bottles, ensuring accuracy, efficiency, and aesthetic appeal. By …
Read More »Turn Tables Boosting Efficiency : Streamlining Material Handling and Process Optimization
Turn Tables Boosting Efficiency: Streamlining Material Handling and Process Optimization Turn tables, also known as rotary tables or revolving tables, are versatile and innovative equipment that revolutionize material handling and process optimization. These rotating platforms enable smooth and controlled movement of products, components, or equipment, enhancing efficiency, reducing manual labor, …
Read More »Unlocking Success with User Requirement Specification: Coating Machine URS
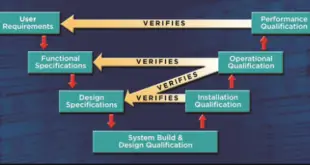
Unlocking Success with User Requirement Specification: Coating Machine URS In any project or system development, understanding and capturing user requirements accurately is crucial for success. User Requirement Specification (URS) is a structured document that serves as a foundation for defining and documenting user needs, expectations, and functional requirements. It forms …
Read More »Optimal HVAC Systems for Non-Sterile Pharmaceutical Environments: Ensuring Quality and Compliance
Optimal HVAC Systems for Non-Sterile Pharmaceutical Environments: Ensuring Quality and Compliance In the pharmaceutical industry, maintaining a controlled environment is crucial to ensure product quality, safety, and regulatory compliance. HVAC (Heating, Ventilation, and Air Conditioning) systems play a vital role in non-sterile pharmaceutical facilities by providing appropriate temperature, humidity, and …
Read More »Temperature mapping of storage areas: Ensuring Reliable Environmental Control and Compliance
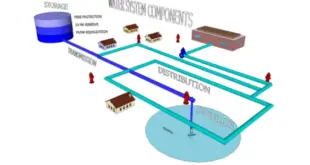
Temperature mapping of storage areas: Ensuring Reliable Environmental Control and Compliance In various industries, maintaining proper temperature control within critical areas is essential to ensure product quality, integrity, and regulatory compliance. Temperature mapping of storage areas is a systematic process that involves monitoring and analyzing temperature distribution within a defined …
Read More »Vial Washing Machine: Streamlining Sterile Pharmaceutical Packaging
Vial Washing Machine: Streamlining Sterile Pharmaceutical Packaging In the pharmaceutical industry, maintaining the integrity and cleanliness of packaging materials is crucial for ensuring the safety and efficacy of drugs. Vial washing machines play a vital role in the production of sterile pharmaceuticals by efficiently cleaning and sanitizing vials before they …
Read More »Design Qualification of Gelatin Colour Mixer
Design Qualification of Gelatin Colour Mixer OBJECTIVE: To design, engineer and supply the Gelatin Colour Mixer and to provide assurance that the machine is manufactured and it complies with the Scope of Supply. To prove that each operation proceeds as per the design qualification and the tolerances prescribed there in …
Read More »Installation Qualification of Gelatin Storage Vessel
Installation Qualification of Gelatin Storage Vessel PURPOSE: To describe the Installation Qualification of Gelatin Storage Vessel its accessories and to define the Specification of the system in order to: Ensure that the equipment meets the specification as per Design Qualification. Aid verification of the installation as per equipment general arrangement …
Read More »Principles of HVAC Duct Design in Pharma Industry
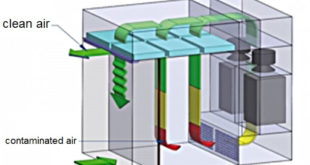
Principles of HVAC Duct Design in the Pharma Industry How Does a Duct System Work The duct, or air distribution, system used in cooling and heating your area is a collection of tubes that distributes the heated or cooled air to the different rooms in the Pharma Industry. This branching …
Read More »Process Validation: General Principles (USFDA) in Pharma Industry
Process Validation: General Principles (USFDA) in Pharma Industry This guidance incorporates principles and approaches that all manufacturers can use to validate manufacturing processes. FDA encourages the use of modern pharmaceutical development concepts, quality risk management, and quality systems at all stages of the manufacturing process lifecycle. The lifecycle concept links …
Read More »Establishment of a Control Procedure in Pharma for Technical Equipment, including related Utilities, Computerized Systems and Facilities used in the Manufacture of Pharmaceuticals
Establishment of a Control Procedure in Pharma for Technical Equipment, including related Utilities, Computerized Systems and Facilities used in the Manufacture of Pharmaceuticals A formal change control system should be established to evaluate all changes that may affect production and control of the intermediate or API. There should be a …
Read More »Qualification of existing facilities, systems, equipment and utilities
Qualification of existing facilities, systems, equipment, and utilities An existing facility has a mixture of new fully qualified equipment (DQ, IQ, OQ, PQ) and partially qualified/ unqualified equipment. Through many years of use, it can usually be shown that facilities, systems, equipment, and utilities can function to varied requirements. Nevertheless, …
Read More »All Post URL of Drugs formulations
All Post URL of Drugs formulations Tablets MFR of Albendazole Tablets – (drugsformulations.com) MFR of Alfacalcidol & Calcium Carbonate Tablets (drugsformulations.com) MFR of Sildenafil Citrate Tablets (drugsformulations.com) MFR of Paracetamol & Dicyclomine HCl Tablets – (drugsformulations.com) MFR of Dextromethorphan, Bromhexine, Chlorpheniramine Maleate & Guaifenesin Tablets (drugsformulations.com) Dextromethorphan (drugsformulations.com) Spiramycin Tablet (drugsformulations.com) MFR of Propranolol Hydrochloride …
Read More »Qualification & Validation of Equipment, Systems, Utilities in Pharmaceuticals
Qualification & Validation of Equipment Equipment qualification as required by the FDA requires verification documentation to start with the Validation Master Plan (VMP) and flow through a series of documents that define the scope and tasks required to successfully execute your equipment qualification task. The VMP dictates the actions that all persons involved …
Read More »PERFORMANCE QUALIFICATION PROTOCOL PURIFIED WATER SYSTEM
PERFORMANCE QUALIFICATION PROTOCOL PURIFIED WATER SYSTEM OBJECTIVE To describe the Performance Qualification procedure to be used during validation of purified water system in order to: a) ensure the system reproducibility over an appropriate time period as per user requirement specifications. b) ensure that the system is showing consistency in producing …
Read More »Guidance Document Cleaning Validation
Guidance Document Cleaning Validation Scope Introduction Principles Validation of Cleaning Processes Equipment and Personnel Microbiological Considerations Documentation Analytical Methods Sampling, Rinsing, Rinse Samples and Detergents Establishment of Limits Change Control/revalidation References 3.1 The objective of the cleaning validation is to verify the effectiveness of the cleaning procedure for removal of …
Read More »Non-sterile process validation
Non-sterile process validation Process validation data should be generated for all products to demonstrate the adequacy of the manufacturing process at each site of manufacture. The validation should be carried out in accordance with good manufacturing practices (GMP) and data should be held at the manufacturing location and made available …
Read More »Performace Qualification Protocol of Dispensing Booth
Performance Qualification Protocol of Dispensing Booth
Read More »Pharma Factory Acceptance Test for Automatic Strip Packing Machine
Factory Acceptance Test for Automatic Strip Packing Machine machine name AUTOMATIC STRIP PACKING MACHINE Client Name & Address M/S. document name factory acceptance test PROTOCOL no. SCT/FAT/000 Revision no. 00 MACHINE SR. NO. Table of Contents Sr. No. Title Page No. 1.0 Purpose 2.0 Scope 3.0 Responsibilities 4.0 System description …
Read More »