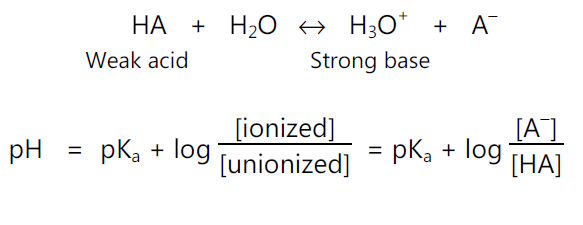Determination of equilibrium solubility of a drug
Determination of equilibrium solubility of a drug:
The drug is dispersed in a solvent. The suspension is agitated at a steady temperature.
Samples of the suspension are withdrawn as a function of time, clarified by centrifugation,and assayed to establish a plateau concentration.
Solvents taken:
(i) 0.9% NaCl at room temperature
(ii) 0.01 M HCl at RT
(iii) 0.1 M HCl at RT
(iv) 0.1 M NaOH at RT
(v) At pH 7.4 buffer at 37oC
Drug concentration is determined by the following analytical methods:
(i) HPLC
(ii) UV –Spectroscopy
(iii) Fluorescence Spectroscopy
(iv) Gas Chromatography
Solubility depends on
(i) pH
(ii) Temperature
(iii) Ionic strength
(iv) Buffer concentration
Significance:
(i) A drug for oral administrative should be examined for solubility in an isotonic saline solution and acidic pH. This solubility data may provide the dissolution profile invivo.
(ii) Solubility in various mediums is useful in developing suspension or solution toxicologic and pharmacologic studies.
(iii) Solubility studies identify those drugs with a potential for bioavailability problems.
E.g. Drug having limited solubility (7 %) in the fluids of GIT often exhibit poor or erratic absorption unless dosage forms are tailored for the drug.
pKa Determination:
• When a weakly acidic or basic drug partially ionizes in GI fluid, generally, the unionized molecules are absorbed quickly.
• Handerson-Hasselbach equation provides an estimate of the ionized and unionized drug concentration at a particular pH.
• For acidic drugs: e.g.
Methods of Determination of pKa of a Drug:
(i) Detection of spectral change by UV or visible spectroscopy at a range of pH:
Advantage: Dilute aqueous solutions can be analyzed by this method.
(ii) Potentiometric titration:
Advantage: Maximum sensitivity for compounds with pKa in the range of 3 to 10.
Disadvantage: This method is unsuccessful for candidates where precipitation of the unionized forms occurs during titration. To prevent precipitation a co-solvent e.g. methanol or dimethylsulfoxide (DMSO) can be incorporated.
• Difference of solubility at various pH.
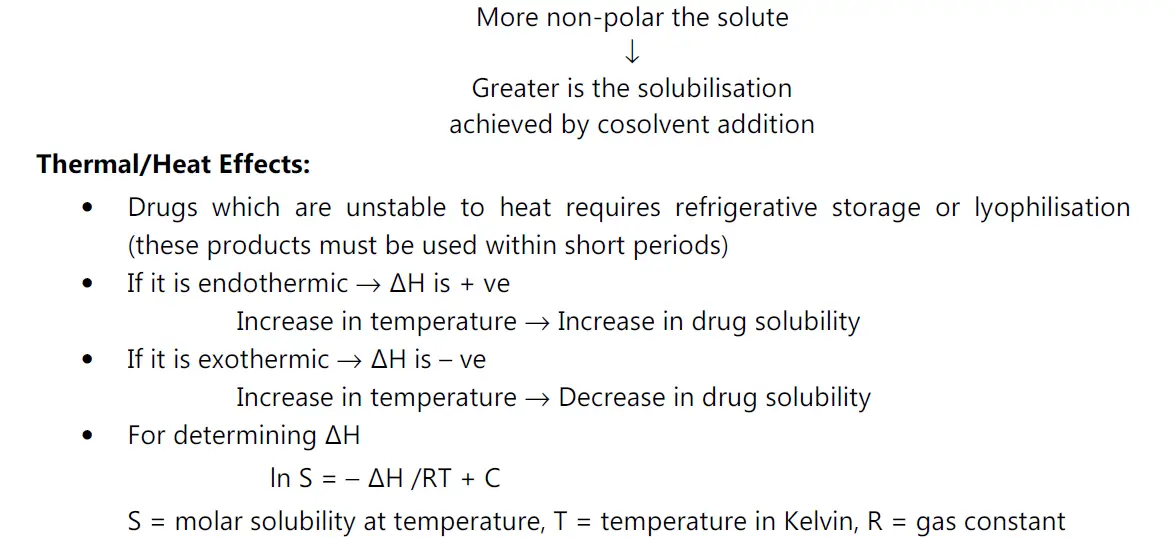
(iii) Effect of temperature on stability:
• Heat of solution, ΔHS represents the heat released or absorbed when a mole of solute is dissolved in a large quantity of solvent.
Significance:
• Most normally, the solubility process is endothermic, e.g. non-electrolytes,unionized forms of weak acids and bases. if ΔHs is positive, solubility increases if temperature increases.
• Solutes that are ionized when dissolved releases heat, the process is exothermic, ΔHs is negative solubility increases as temperature decreases.
(iv) Solubilization:
• For drug candidates with poor water solubility, preformulation studies should include limited experiments to identify the possible mechanisms for solubilisation.
• Means of increasing the solubility are:
(i) Addition of a cosolvent to the aqueous system e.g. ethanol, propylene glycol and glycerine.
MOA: These co-solvents disrupt the hydrophobic interactions of water at the non-polar solute / water interfaces.
(ii) Solubilisation in micellar solutions such as 0.01 M Tween 20 solution.
(iii) Solubilisation by forming molecular complexes e.g. benzoic acid forms complex with caffeine.
(v) Partition coefficient:
• Partition coefficient is defined, as the ratio of un-ionized drug concentrations between the organic and aqueous phases, at equilibrium.
• Generally, 2-octanol and chloroform are taken as the oil phase. m equilibrium
Significance: Drug molecules having higher KO/W will cross the lipid cell membrane.
(vi) Dissolution:
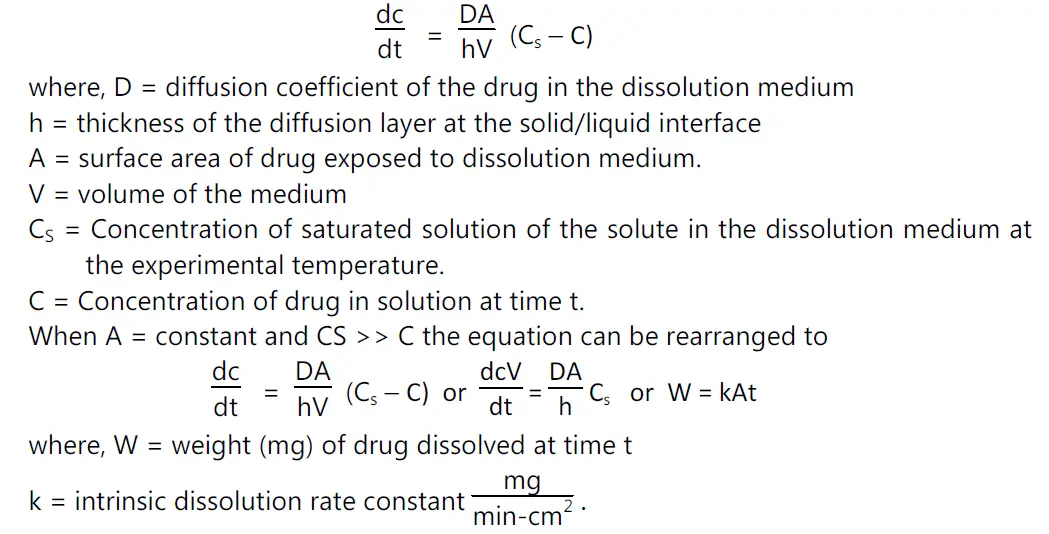
• The dissolution rate of a drug substance in which surface area is constant during disintegration is described by the modified Noyes-Whitney equation
Determination of k:
• Pure drug powder is punched in a die and punch apparatus to give a uniform cylindrical shape. The tablet is covered with wax in all sides. One circular face is exposed to the dissolution medium. Thus, as dissolution precedes, the area, A, remains constant.
• Time to time dissolution medium is taken out and fresh medium added to the chamber.
• With two types of assembly, the experiments can be carried out.
pH and pKa Solubility Profile:
• pKa Determination: The Henderson – Hasseslebach equation provides an estimate of the ionized and unionized drug concentration at a particular pH.
For acidic drugs,
pH = pKa + log (ionized drug / un-ionized drug)
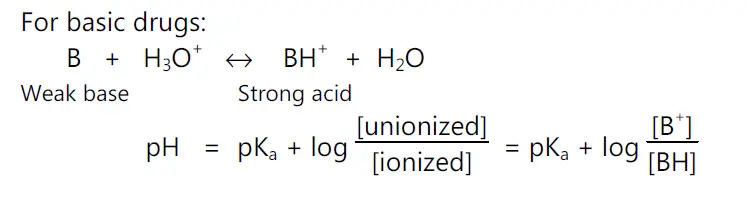
For basic drugs,
pH = pKa + log (unionized drug / ionized drug)
• Buffers, temperature, ionic strength and cosolvent can affect the pKa value.
• Potentiometric titration offers maximum sensitivity for compounds with pKa values in the range of 3-10.
Solubilization:
• Solubilization is increased by cosolvent addition.
• E.g. Propylene glycol solubilizes drug molecules by disrupting the hydrophobic interactions of water.
Particle size:
• Fine particle characterization is very important property and here smallest particle should be tested to facilitate homogeneous sample preparation.
• Coulter Counter Technique: To check particle size and particle volume.
• BET (Brunauer, Emmet, Teller) nitrogen absorption apparatus: Measurement of surface area.
• SEM (Scanning Electron Microscopy): To check surface morphology.
Other Related Post – Powder Particle Size Determination Methods for tablets